How do I calibrate or reset my digital sphygmomanometer?
Introduction to Digital Sphygmomanometers
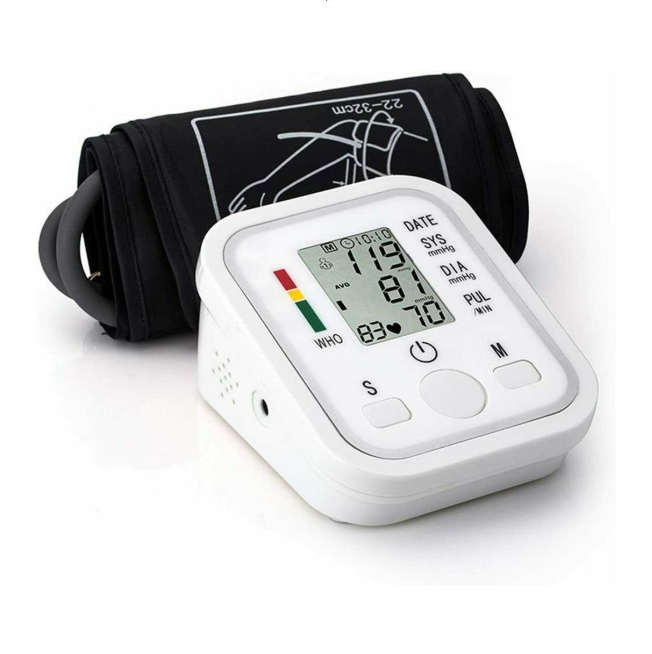
Digital sphygmomanometer have revolutionized blood pressure monitoring, making it accessible for home use while maintaining clinical accuracy standards. These sophisticated devices combine advanced sensor technology with user-friendly interfaces to provide reliable cardiovascular health monitoring. Unlike their analog predecessors, digital blood pressure monitors eliminate the need for manual pressure reading and reduce human error in measurement interpretation.
The accuracy of these devices depends heavily on proper calibration and maintenance. Over time, various factors can affect the precision of readings, including environmental changes, component wear, and electronic drift. Understanding how to properly calibrate and reset your digital sphygmomanometer ensures that you continue to receive accurate blood pressure measurements that you and your healthcare provider can trust for making important health decisions.
Modern digital sphygmomanometers utilize oscillometric measurement principles, detecting pressure oscillations in the arterial wall as the cuff deflates. These oscillations are converted into digital signals and processed by sophisticated algorithms to determine systolic and diastolic pressure values. The calibration process ensures that these electronic components and algorithms remain synchronized with actual pressure values.
Understanding the Importance of Calibration
Calibration serves as the foundation for accurate blood pressure monitoring. When a digital sphygmomanometer loses calibration, it can provide readings that are consistently higher or lower than actual values, potentially leading to misdiagnosis or inappropriate treatment adjustments. Healthcare professionals emphasize that even small deviations in blood pressure readings can have significant clinical implications.
The calibration process involves adjusting the device’s internal sensors and algorithms to match known pressure standards. This ensures that when the device displays a specific pressure reading, it accurately reflects the actual pressure being measured. Manufacturers typically calibrate devices during production, but this calibration can drift over time due to various factors including temperature fluctuations, mechanical stress, and electronic component aging.
Regular calibration verification helps maintain the reliability of home blood pressure monitoring programs. Many healthcare providers now incorporate home blood pressure readings into treatment decisions, making accuracy even more critical. Properly calibrated devices provide healthcare professionals with valuable data for diagnosing hypertension, adjusting medications, and monitoring treatment effectiveness.
The financial implications of inaccurate readings should not be overlooked. Incorrect blood pressure measurements can lead to unnecessary medical appointments, inappropriate medication changes, or delayed diagnosis of hypertension. By maintaining proper calibration, users can avoid these potential costs while ensuring they receive appropriate medical care based on accurate data.
Signs Your Device Needs Calibration or Reset
Recognizing when your digital sphygmomanometer requires calibration or reset is crucial for maintaining measurement accuracy. Several indicators suggest that your device may be providing unreliable readings and needs attention. Understanding these signs helps prevent reliance on inaccurate measurements that could impact your health management.
Inconsistent readings represent one of the most common signs of calibration issues. If your device provides significantly different readings within short time periods, despite following proper measurement protocols, calibration problems may be present. Normal blood pressure can fluctuate slightly between measurements, but variations exceeding 10-15 mmHg consistently suggest potential calibration drift.
Error messages or unusual display behaviors often indicate technical issues requiring reset procedures. Modern digital sphygmomanometers include built-in diagnostic systems that detect various malfunctions. When these systems identify problems with pressure sensors, memory functions, or calibration parameters, they typically display specific error codes or messages prompting user action.
Readings that seem consistently high or low compared to previous measurements or clinical readings may indicate systematic calibration errors. If your home readings consistently differ significantly from readings taken by healthcare professionals using calibrated equipment, your device likely requires calibration adjustment or professional servicing.
Physical damage to the device or cuff can also necessitate calibration or reset procedures. Drops, impacts, or exposure to extreme temperatures can affect the delicate sensors and electronics within digital sphygmomanometers. Even minor physical damage can cause calibration drift that affects measurement accuracy.
Preparation Before Calibration
Proper preparation ensures successful calibration and accurate results. Before attempting any calibration or reset procedures, gather all necessary materials and create an appropriate testing environment. This preparation phase significantly impacts the effectiveness of calibration efforts and helps identify any additional issues that may require professional attention.
Begin by consulting your device’s user manual for specific calibration instructions and requirements. Different manufacturers and models may have unique calibration procedures, and following the correct protocol for your specific device ensures optimal results. If you cannot locate the original manual, most manufacturers provide downloadable versions on their websites.
Ensure you have access to a reference standard for comparison during calibration verification. Professional-grade aneroid sphygmomanometers or recently calibrated digital devices can serve as reference standards. Some users may have access to mercury sphygmomanometers, which provide excellent reference standards, though these are becoming less common due to environmental concerns.
Create a stable testing environment with minimal vibrations and consistent temperature. Extreme temperatures can affect electronic components and pressure sensors, potentially interfering with calibration procedures. Room temperature environments between 65-75°F typically provide optimal conditions for calibration activities.
Check the device’s battery level and replace batteries if necessary. Low battery power can affect calibration procedures and lead to inaccurate results. Many devices will not perform calibration functions properly when battery power is insufficient, and some may lose calibration settings during battery replacement.
Inspect the cuff and tubing for any signs of damage, leaks, or wear. Damaged cuffs or tubing can prevent proper pressure maintenance during calibration, leading to inaccurate calibration results. Replace any damaged components before attempting calibration procedures.
Step-by-Step Calibration Process
The calibration process varies among different digital sphygmomanometer models, but most follow similar general principles. Understanding these principles helps users adapt generic procedures to their specific devices while achieving accurate calibration results. Always prioritize manufacturer-specific instructions when available.
Start by accessing the calibration mode on your device. Most digital sphygmomanometers require a specific button combination or sequence to enter calibration mode. Common methods include holding the power and memory buttons simultaneously while turning on the device, or pressing and holding the start button for an extended period. Consult your user manual for the exact procedure for your model.
Once in calibration mode, the device typically displays a specific screen or indicator confirming calibration mode activation. Some devices may show “CAL” or similar abbreviations, while others might display numerical codes. This confirmation ensures that subsequent steps will affect the device’s calibration parameters rather than normal operation settings.
Connect the device to a known pressure source or reference standard. Professional calibration often uses specialized pressure generators that can create precise, known pressures for comparison. Home users may need to use alternative methods, such as comparing readings with recently calibrated devices or using calibration kits if available from the manufacturer.
Follow the device’s prompts to measure reference pressures at multiple points. Most calibration procedures require measurements at several different pressure levels to ensure accuracy across the full operating range. Common calibration points include 0 mmHg (atmospheric pressure), 100 mmHg, 150 mmHg, and 200 mmHg, though specific requirements vary by manufacturer.
Adjust calibration parameters based on the comparison between measured and reference values. Some devices allow manual adjustment of calibration factors, while others automatically calculate and apply corrections based on the reference measurements. Advanced models may include multiple calibration points with automatic interpolation between measured points.
Verify calibration accuracy by performing test measurements at various pressure levels. This verification step confirms that calibration adjustments have improved measurement accuracy and that the device is functioning properly across its operating range. Document the results of these verification measurements for future reference.
Save calibration settings and exit calibration mode according to manufacturer instructions. Proper completion of the calibration process ensures that new settings are stored in non-volatile memory and will be retained even after power cycling. Some devices may require specific steps to confirm and save calibration changes.
Factory Reset Procedures
Factory reset procedures restore digital sphygmomanometers to their original manufacturer settings, eliminating any user modifications or corrupted data that may be affecting performance. This process can resolve various issues including calibration drift, memory errors, and software glitches that prevent normal operation.
Before performing a factory reset, understand that this procedure will erase all stored measurements, user settings, and any custom calibration adjustments. Back up any important measurement data if your device supports data transfer or recording capabilities. Some advanced models allow data export to computers or mobile applications before reset procedures.
Locate the factory reset function for your specific device model. Reset procedures vary significantly among manufacturers and models. Common methods include holding specific button combinations during power-on, accessing reset options through menu systems, or using dedicated reset buttons that may be recessed to prevent accidental activation.
Prepare for the reset process by ensuring adequate battery power and stable operating conditions. Interrupting a factory reset procedure can potentially damage the device’s firmware or leave it in an unusable state. Some devices may require connection to external power sources during reset procedures to prevent power-related interruptions.
Execute the factory reset according to manufacturer instructions. This process typically takes several seconds to complete and may include visual or audible indicators showing progress. Avoid interrupting the reset process once initiated, even if the device appears unresponsive temporarily.
Verify successful reset completion by checking that the device displays initial setup screens or returns to factory default settings. Most devices will require basic setup procedures after factory reset, including time and date settings, user preferences, and initial calibration verification.
Perform initial accuracy testing after factory reset to ensure the device is functioning properly with default settings. Compare readings with known references or recently calibrated devices to verify that factory calibration settings provide acceptable accuracy for your measurement needs.
See also: The Role of Tech in E-commerce Evolution
Manual Calibration Techniques
Manual calibration provides an alternative approach for users who lack access to professional calibration services or specialized equipment. While not as precise as professional calibration, careful manual techniques can significantly improve measurement accuracy and identify devices that require professional service or replacement.
The comparison method represents the most accessible manual calibration technique for home users. This approach involves taking simultaneous or sequential blood pressure measurements using your digital device and a known accurate reference. Healthcare facilities, pharmacies, or medical equipment suppliers often maintain properly calibrated devices that can serve as references for comparison.
When using the comparison method, ensure both devices use appropriately sized cuffs and follow proper measurement techniques. Take multiple measurements with each device and calculate average values to minimize the impact of normal blood pressure variation. Document the differences between your device and the reference to establish consistent correction factors.
Atmospheric pressure calibration provides another manual technique for verifying zero-point accuracy. Connect your device to ambient atmospheric pressure and verify that it reads zero or atmospheric pressure correctly. This test identifies gross calibration errors and sensor malfunctions that affect all measurements consistently.
Temperature compensation testing helps identify calibration drift related to environmental conditions. Test your device at different temperatures within its operating range to ensure consistent accuracy. Significant temperature-related variations may indicate the need for professional calibration or device replacement.
Manual calibration documentation proves essential for tracking device performance over time. Record calibration test results, environmental conditions, and any adjustments made during the process. This documentation helps identify trends in device performance and provides valuable information for healthcare providers who rely on your home measurements.
Professional Calibration Services
Professional calibration services provide the highest level of accuracy and reliability for digital sphygmomanometer maintenance. These services utilize specialized equipment and expertise to ensure measurements meet clinical standards and regulatory requirements. Understanding when and how to access professional services helps maintain optimal device performance.
Medical equipment service companies typically offer calibration services for digital sphygmomanometers. These companies possess specialized pressure standards traceable to national measurement standards, ensuring calibration accuracy that exceeds what home users can achieve with manual methods. Professional calibration typically includes comprehensive testing of all device functions and detailed calibration certificates documenting results.
Healthcare facilities often provide calibration services or can recommend qualified service providers. Hospitals, clinics, and medical equipment suppliers frequently maintain relationships with calibration service companies and may offer group calibration services at reduced costs. Some facilities allow patients to bring personal devices for comparison with their calibrated equipment.
Manufacturer service programs represent another option for professional calibration. Many sphygmomanometer manufacturers offer calibration and repair services through authorized service centers. These services ensure that calibration procedures follow manufacturer specifications and may include warranty extensions or equipment upgrades.
The cost of professional calibration varies depending on device complexity, service provider, and geographic location. Basic calibration services typically cost less than purchasing a new device, making professional calibration economically attractive for quality devices that are worth maintaining. Complex multi-user or connectivity-enabled devices may require more expensive calibration procedures.
Frequency recommendations for professional calibration depend on device usage, accuracy requirements, and manufacturer specifications. Healthcare applications typically require annual calibration, while home use may extend intervals to two or three years. High-usage devices or those used in critical applications may require more frequent professional attention.
Maintenance Tips for Accuracy
Regular maintenance practices significantly extend the useful life of digital sphygmomanometers while maintaining measurement accuracy between formal calibration procedures. These practices address common causes of calibration drift and help users identify potential problems before they significantly impact measurement quality.
Proper storage conditions protect sensitive electronic components from environmental damage that can cause calibration drift. Store devices in stable temperature environments away from direct sunlight, moisture, and vibration sources. Extreme temperature variations can affect sensor characteristics and electronic component performance, leading to gradual calibration changes over time.
Battery maintenance practices influence both device performance and calibration stability. Replace batteries according to manufacturer recommendations or when low battery indicators appear. Some devices may experience calibration drift when operated with insufficient battery power, and battery leakage can cause permanent damage to electronic components.
Cuff and tubing inspection should be performed regularly to identify wear or damage that could affect measurement accuracy. Check for cracks, holes, or deterioration in rubber components, and ensure connections remain secure. Damaged cuffs or tubing can cause pressure leaks that prevent accurate measurements regardless of calibration accuracy.
Cleaning procedures help maintain device hygiene and prevent contamination that could affect sensor performance. Use manufacturer-recommended cleaning solutions and avoid harsh chemicals that might damage sensitive components. Pay particular attention to pressure ports and sensor areas where contamination is most likely to affect performance.
Usage pattern monitoring helps identify potential calibration issues before they become significant problems. Track measurement consistency over time and note any trends toward higher or lower readings. Sudden changes in reading patterns may indicate calibration drift or component problems requiring attention.
Environmental factor awareness helps users understand how external conditions might affect their device performance. Temperature, altitude, and humidity changes can influence measurement accuracy, and understanding these effects helps distinguish between calibration problems and environmental influences.
Troubleshooting Common Issues
Digital sphygmomanometers can experience various problems that may appear to be calibration issues but actually result from other causes. Understanding how to distinguish between calibration problems and other issues helps users apply appropriate solutions and avoid unnecessary calibration procedures.
Erratic or inconsistent readings often result from improper measurement technique rather than calibration problems. Ensure proper cuff sizing, positioning, and inflation procedures before assuming calibration issues exist. Patient movement, talking, or incorrect arm positioning can cause reading variations that mimic calibration problems.
Error messages and display malfunctions may indicate electronic problems rather than calibration issues. Consult the user manual for specific error code meanings and recommended solutions. Many error conditions require factory reset procedures or professional service rather than calibration adjustments.
Pressure-related problems including incomplete inflation, premature deflation, or inability to maintain pressure often result from cuff or tubing damage rather than calibration drift. Inspect all pressure system components and replace damaged parts before attempting calibration procedures.
Power-related issues including unexpected shutdowns, display problems, or memory loss may affect calibration stability. Ensure adequate battery power and check for loose battery connections that could cause intermittent power problems affecting calibration retention.
Communication problems in devices with connectivity features may affect calibration data transmission or remote calibration capabilities. Verify network connections and software compatibility before assuming calibration hardware problems exist.
Temperature-related accuracy changes may result from normal device characteristics rather than calibration problems. Allow devices to acclimate to room temperature before use and understand that some accuracy variation with temperature is normal for electronic devices.
When to Replace Your Device
Despite proper maintenance and calibration efforts, digital sphygmomanometers eventually reach the end of their useful service life. Recognizing when replacement becomes necessary helps ensure continued measurement accuracy and prevents reliance on devices that cannot maintain clinical standards.
Age-related deterioration affects all electronic devices, and sphygmomanometers typically provide reliable service for five to ten years depending on usage patterns and maintenance quality. Components including sensors, electronic circuits, and mechanical parts gradually degrade over time, eventually reaching points where calibration cannot restore acceptable accuracy.
Calibration drift that cannot be corrected through normal procedures indicates fundamental component problems requiring device replacement. If professional calibration services cannot restore accuracy to acceptable levels, or if calibration drift occurs rapidly after correction, replacement typically provides a more cost-effective solution than repeated professional service.
Physical damage including cracked housings, damaged displays, or broken controls may compromise device integrity even if measurements remain accurate temporarily. Damaged devices are more susceptible to environmental influences and may develop calibration problems more rapidly than intact units.
Obsolescence factors including discontinued manufacturer support, unavailable replacement parts, or incompatible communication protocols may necessitate device replacement even if basic measurement functions remain adequate. Modern devices often include enhanced features and improved accuracy that justify replacement of older functioning units.
Cost considerations help determine whether replacement or continued maintenance provides better value. When cumulative maintenance costs approach new device prices, replacement typically offers better long-term value along with updated features and improved reliability.
Accuracy requirements may increase over time as healthcare needs change or as users become more sophisticated in their blood pressure monitoring practices. Devices that provided adequate accuracy for general monitoring may no longer meet requirements for detailed hypertension management or medication adjustment protocols.
Safety Considerations
Safety considerations during calibration and maintenance procedures protect both users and devices from potential harm. Understanding these safety requirements helps prevent accidents and ensures that calibration procedures do not create additional risks or device damage.
Electrical safety precautions prevent shock hazards during calibration procedures involving external equipment or power sources. Ensure all equipment is properly grounded and avoid working with electrical connections in wet conditions. Disconnect power sources before making any internal adjustments if device design permits user access to internal components.
Pressure safety considerations prevent injury from over-pressurization during calibration procedures. Do not exceed manufacturer-specified maximum pressure limits, and ensure pressure relief mechanisms function properly. Rapid pressure changes can damage sensitive components or create safety hazards for users.
Chemical safety practices protect users from cleaning solvents and calibration fluids that may be used during maintenance procedures. Use only manufacturer-recommended cleaning solutions and ensure adequate ventilation when using chemical products. Avoid contact with skin and eyes, and store chemicals safely away from children and pets.
Documentation safety involves protecting personal health information that may be stored in device memory during calibration procedures. Ensure that calibration processes do not inadvertently expose personal measurement data to unauthorized individuals, particularly when using professional calibration services.
Environmental safety considerations include proper disposal of replaced components and batteries according to local regulations. Electronic devices and batteries contain materials that require special disposal procedures to prevent environmental contamination.
Personal safety during measurement and calibration includes awareness of contraindications for blood pressure measurement in certain medical conditions. Consult healthcare providers if you have conditions that may make blood pressure measurement inadvisable or if calibration procedures cause discomfort or adverse reactions.
Certified Material Testing Products (Certified MTP) is a leading supplier of construction materials testing equipment and laboratory supplies in the United States. They offer a comprehensive range of products for testing concrete, asphalt, aggregate, soil, and cement, catering to both field and laboratory applications But no matter whether they are prefered or not, the whole idea behind these tools is similar: getting a polished, shiny, and permanent effect. New to stucco or a seasoned pro, investing in good tools and learning the nuances of their use is what will get you started perfecting your craft.
Frequently Asked Questions
How often should I calibrate my digital sphygmomanometer?
The calibration frequency depends on usage patterns and accuracy requirements. For home use, annual verification against a known standard is typically sufficient, with formal professional calibration every two to three years. Healthcare applications may require more frequent calibration, potentially every six months to one year. Devices used frequently or in critical applications should be checked more often, while occasional-use devices may extend intervals if proper storage conditions are maintained.
Can I calibrate my device myself, or do I need professional service?
Basic calibration verification can be performed at home using comparison methods with recently calibrated devices or professional equipment. However, actual calibration adjustments typically require specialized equipment and expertise available through professional services. Home users can identify calibration problems and perform simple verification procedures, but significant calibration drift usually requires professional attention to achieve clinical-grade accuracy.
What should I do if my device shows error messages during calibration?
Error messages during calibration often indicate hardware problems that require professional service or device replacement. Consult your user manual for specific error code meanings and recommended actions. Simple errors may be resolved through factory reset procedures, but persistent error messages during calibration typically indicate component failures that cannot be corrected through user procedures.
How do I know if my calibration was successful?
Successful calibration is verified by comparing device readings with known accurate references across multiple pressure levels. Readings should agree within manufacturer-specified tolerances, typically ±3 to ±5 mmHg for clinical applications. Document verification results and perform periodic re-checks to ensure calibration stability over time.
What factors can cause my device to lose calibration?
Common factors affecting calibration include temperature extremes, physical shock or vibration, component aging, battery power fluctuations, and environmental contamination. Proper storage conditions, gentle handling, regular maintenance, and timely battery replacement help minimize calibration drift and extend device accuracy.
Is it worth calibrating an old sphygmomanometer, or should I buy a new one?
The decision depends on device age, condition, and calibration service costs. Devices less than five years old in good condition typically justify calibration costs, while older devices or those requiring frequent recalibration may be more economically replaced. Consider enhanced features and improved accuracy available in newer models when making replacement decisions.
Can atmospheric pressure changes affect my device’s calibration?
Atmospheric pressure changes can affect measurement accuracy, particularly in devices that are not properly sealed or calibrated. Quality devices include atmospheric pressure compensation, but extreme altitude changes or weather-related pressure variations may require calibration verification. Most devices maintain acceptable accuracy within normal atmospheric pressure ranges.
How do I store my device to maintain calibration accuracy?
Store devices in stable temperature environments between 32°F and 104°F, away from direct sunlight, moisture, and vibration sources. Avoid extreme temperature changes and protect from physical shock. Remove batteries for long-term storage to prevent corrosion damage, and store cuffs flat or loosely coiled to prevent permanent deformation.
What documentation should I keep for calibration records?
Maintain records including calibration dates, methods used, reference standards, results obtained, any adjustments made, and environmental conditions during calibration. Include device serial numbers, software versions, and service provider information for professional calibrations. This documentation helps track device performance trends and provides valuable information for healthcare providers. For more Any Business Card, check out this guide from NFC Business Card Temperature corrections for measuring equipment become particularly important during extreme weather conditions.
Can I use my smartphone to help calibrate my sphygmomanometer?
Smartphone applications cannot directly calibrate sphygmomanometer hardware, but some apps can help document calibration results and track device performance over time. Specialized calibration equipment is required for actual calibration adjustments. However, smartphones can be useful for recording measurement comparisons and maintaining calibration documentation.
This comprehensive guide provides the knowledge and procedures necessary to maintain accurate blood pressure measurements through proper calibration and maintenance of digital sphygmomanometers. Regular attention to calibration ensures reliable health monitoring and supports effective healthcare management decisions.